A striking feature of nervous systems is their enormous cellular complexity. To understand how the nervous system develops, one needs to understand the gene regulatory mechanisms that result in the expression of neuron type-specific gene batteries and which determine the functional properties of a mature neuron. We use the nervous system of Caenorhabditis elegans (Figure 1) as a model system to gain molecular and mechanistic insights into how a nervous system develops and how individual neuron types differentiate.
(a) The concept of terminal selector regulons
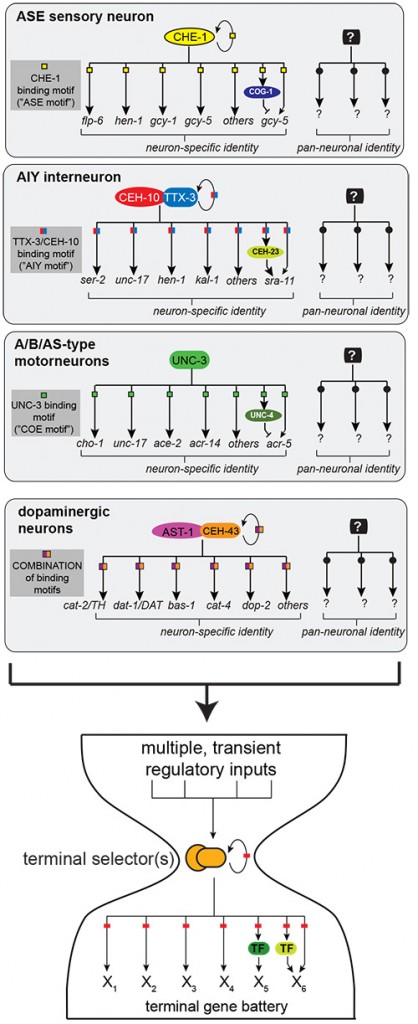
We study the generation of neuronal diversity from an angle not commonly employed. We reason that the underlying basis of the functional and anatomical diversity of cell types in the nervous system is the differential expression of neuron-type specific gene batteries, composed of terminal differentiation genes whose products define the specific properties of a mature neuron throughout its lifetime (e.g. neurotransmitter-synthesizing enzymes, transporters, receptors)[1]. Thus, one approach to understand the generation of neuronal diversity is a “bottom-up” approach that ignores the commonly studied early or intermediary steps of neuronal development but focuses on the terminal step of this process, i.e. the regulation of terminal gene batteries. To this end, we have defined the molecular signatures of individual, terminally differentiated neuron types and decoded the cis-regulatory control regions of terminal gene batteries through systematic mutational analysis of reporter genes in the context of transgenic animals[2-5]. This mutational dissection revealed what we consider to be an important, far-reaching and non-obvious principle: co-regulation (Figure 2). That is, entire ensembles of terminal differentiation genes that define the terminal properties – and thus identity and function – of a given neuron type share a common cis-regulatory signature (Figure 2)[2-5].
Through genetic analysis we have identified phylogenetically conserved, neuron-type specific trans-acting factors (or combinations thereof) that act through these cis-regulatory motifs (Figure 2)[2-5]. Loss of these factors leads to a loss of neuron-type specific identity, while pan-neuronal features remain unaffected. We termed these factors “terminal selectors”[6, 7]. Such a regulatory logic was not a given, since transcriptome profiling, conducted by us and others[3, 8], revealed that individual, terminally differentiated neuron types express scores of transcription factors. Terminal gene batteries could have therefore been envisioned to be under complex, piecemeal control, rather than being organized into relatively simple regulons (Figure 2).
As shown in Figure 2, we have probed – and still are continuing to probe – the terminal selector concept in a multitude of C. elegans neuron types.
Extending our studies to chordates, we have provided evidence that this regulatory principle is conserved: Using mouse knockouts, we have shown that the terminal selector controlling dopaminergic neuron differentiation displays the same function in mouse dopaminergic neurons[4] and we have found, in collaboration with M.Levine, that a terminal selector for cholinergic motoneurons, UNC-3, is also functionally conserved in the simple chordate Ciona intestinalis [5]. We are in the process of examining the mouse knockout phenotype of vertebrate homologs of C. elegans terminal selectors.
(b) Deciphering additional regulons:
As shown in Figure 2, terminal selectors regulate the expression of genes that define neuron type-specific properties, but they are not required for the expression of pan-neuronal genes shared by all neuron types. Through a combination of cis-regulatory analysis and automated forward genetic screens, we will investigate the nature of the regulatory programs that control pan-neuronal features and determine whether pan-neuronal programs executed in all neuron types use a co-regulatory strategy or whether regulation occurs in a piecemeal manner. These studies will complement our attempts to understand the regulation of neuron-type identities and will provide a more comprehensive understanding of neuronal development.
(c) Connecting lineage history to terminal differentiation
Investigating how terminal selector gene function is linked to upstream developmental programs, we discovered that cis-regulatory regions of terminal selector loci read out transient regulatory states, characterized by a complex combination of transcriptional and signaling inputs that are specific for the lineage history of a cell[9-11]. These regulatory events converge to transiently initiate terminal selectors, which then “lock in” the terminal state through an autoregulatory feedback loop. We have conceptualized this regulatory topology with the imagery of an hourglass (Figure 2)[7]. In metabolic networks, such regulatory topology has been proposed to endow networks with robustness and evolvability[12] – features that provide an attractive framework for how to think about terminal selector regulons.
(d) Cellular reprogramming
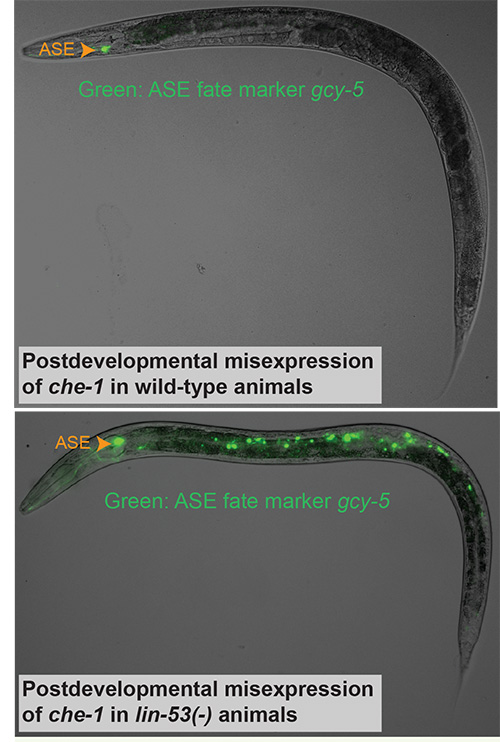
We have asked whether terminal selectors are not only required but also sufficient to drive specific neuronal differentiation programs. This is essentially a cellular reprogramming question, a topic of much recent attention[13]. Misexpression of the terminal selector che-1 in mature animals results in the reprogramming of only a small number of head sensory neurons into ASE neurons, mirroring the temporal and context-dependency of many other prominent developmental control genes[14]. We reasoned that the mechanistic basis of context-dependency lies in the presence of inhibitory, chromatin-based mechanisms. To test this hypothesis, we assessed through an RNAi screen whether eliminating individual chromatin factors would permit CHE-1 to reprogram additional cells. We found that elimination of the PRC2 complex, a histone methyltransferase, allows CHE-1, as well as all other terminal selectors, to convert mitotic germ cells directly into specific neuron types (Figure 3)[14, 15]. These findings underscore the potency of terminal selectors and validate our approach of studying neuronal identity through the analysis of the regulation of terminal differentiation genes.
We have also isolated mutants in which the epidermis of the worm is made “reprogrammable” upon ectopic expression of a terminal selector, suggesting that individual cell types use distinct mechanisms to “shut down” their responsiveness to regulatory inputs. We will further scale up this mutagenesis approach using our technology pipelines described above and pursue an in-depth characterization of these mutants. To circumvent phenotypic pleiotropies, we will harness the specific strength of our genetic system by attempting to isolate conditional alleles.
Apart from possibly providing novel avenues for cellular engineering for various practical applications, we anticipate that our reprogramming studies will provide insights into normal developmental processes. This is evidenced, for example, by our finding that in embryos, upon terminal differentiation of neurons, we do find most neurons types to be easily “reprogrammable” into other neuron types upon ectopic terminal selector expression. However, this “reprogrammability” is lost during postembryonic life, indicating that it is part of normal development to “shut down” responsiveness to regulatory inputs, possibly through the formation of facultative heterochromatin. How facultative heterochromatin formation is targeted to specific chromosomal loci has been a long-standing mystery in the gene regulation field[16] and we anticipate that the genetic screens described above will shed light onto this process.
Figure legends:
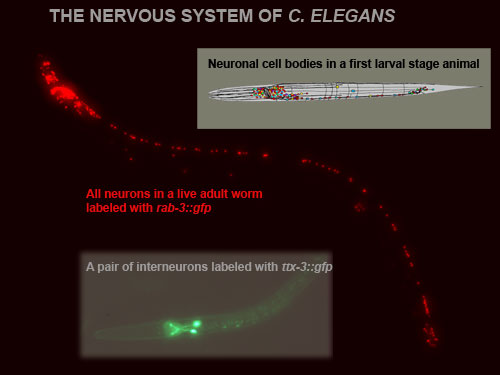
Figure 1: The Nervous system of a C.elegans worm. A first larval stage worm contains 222 neurons (202 somatic, 20 pharyngeal), as shown in the inset. Adult hermaphrodites contain 302 neurons, shown here labeled with a panneuronal marker, rab-3::gfp. Adult males contain another 81 neurons. Many individual neuron types can be labeled with gfp and those transgenic lines serve as invaluable tools to genetically dissect the developmental pathways that generate such neurons.
Figure 2: Terminal selector regulons. Terminal differentiation genes that define the identity of a neuron are co-regulated through a common cis-regulatory signature and cognate trans-acting factor(s) (“terminal selectors”) that act either alone (e.g. CHE-1) or in combination (e.g. TTX-3/CEH-10) and that are continuously expressed throughout the entire life of a neuron. Regulatory events downstream of terminal selectors further diversify terminal differentiation programs. For example, the left and right asymmetric subtype specification of the ASE neuron pair is under control of a complex regulatory network (see left/right asymmetry section) which itself is under control of the terminal selector che-1. Unknown, parallel acting regulatory routines (indicated with “?”) presumably control pan-neuronal features.
Figure 3: Direct reprogramming of germ cells into neurons. The upper panel shows an animal induced to ectopically express the terminal selector che-1 in all cell types, leading to only a small number of head neurons (not visible in this plane of focus) to be reprogrammed toward ASE fate (as shown with a gfp fate marker in this figure). Knock-down of lin-53, a so-called “histone chaperone” and part of the PRC2 complex,allows reprogramming of all mitotic germ cells into mature ASE neurons (lower panel).
Bibliography:
1. Hobert, O., I. Carrera, and N. Stefanakis, The molecular and gene regulatory signature of a neuron. Trends Neurosci, 2010. 33(10): p. 435-45.
2. Wenick, A.S. and O. Hobert, Genomic cis-Regulatory Architecture and trans-Acting Regulators of a Single Interneuron-Specific Gene Battery in C. elegans. Dev Cell, 2004. 6(6): p. 757-70.
3. Etchberger, J.F., et al., The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev, 2007. 21(13): p. 1653-74.
4. Flames, N. and O. Hobert, Gene regulatory logic of dopamine neuron differentiation. Nature, 2009. 458(7240): p. 885-9.
5. Kratsios, P., et al., Coordinated regulation of cholinergic motor neuron traits through a conserved terminal selector gene. Nat Neurosci, 2011. 15(2): p. 205-14.
6. Hobert, O., Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A, 2008. 105(51): p. 20067-71.
7. Hobert, O., Regulation of terminal differentiation programs in the nervous system. Annu Rev Cell Dev Biol, 2011. 27: p. 681-96.
8. Spencer, W.C., et al., A spatial and temporal map of C. elegans gene expression. Genome Res, 2011. 21(2): p. 325-41.
9. Bertrand, V. and O. Hobert, Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans. Dev Cell, 2009. 16(4): p. 563-75.
10. Bertrand, V. and O. Hobert, Wnt asymmetry and the terminal division of neuronal progenitors. Cell Cycle, 2009. 8(13): p. 1973-4.
11. Bertrand, V. and O. Hobert, Lineage programming: navigating through transient regulatory states via binary decisions. Curr Opin Genet Dev, 2010. 20(4): p. 362-8.
12. Csete, M. and J. Doyle, Bow ties, metabolism and disease. Trends Biotechnol, 2004. 22(9): p. 446-50.
13. Cohen, D.E. and D. Melton, Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet, 2011. 12(4): p. 243-52.
14. Tursun, B., et al., Direct conversion of C. elegans germ cells into specific neuron types. Science, 2011. 331(6015): p. 304-8.
15. Patel, T., et al., Removal of Polycomb Repressive Complex 2 Makes C. elegans Germ Cells Susceptible to Direct Conversion into Specific Somatic Cell Types. Cell Rep, 2012. 2(5): p. 1178-86.
16. Trojer, P. and D. Reinberg, Facultative heterochromatin: is there a distinctive molecular signature? Mol Cell, 2007. 28(1): p. 1-13.